Supramolecular Cluster Catalysis: Benzene Hydrogenation Catalyzed by a Cationic Triruthenium Cluster under Biphasic Conditions
Georg Süss-Fink Prof. Dr.
Institut de Chimie, Université de Neuchâtel Case postale 2, 2007 Neuchâtel, Switzerland, Fax: (+41) 32-718-2511
Search for more papers by this authorMatthieu Faure
Institut de Chimie, Université de Neuchâtel Case postale 2, 2007 Neuchâtel, Switzerland, Fax: (+41) 32-718-2511
Search for more papers by this authorThomas R. Ward Prof. Dr.
Institut de Chimie, Université de Neuchâtel Case postale 2, 2007 Neuchâtel, Switzerland, Fax: (+41) 32-718-2511
Search for more papers by this authorGeorg Süss-Fink Prof. Dr.
Institut de Chimie, Université de Neuchâtel Case postale 2, 2007 Neuchâtel, Switzerland, Fax: (+41) 32-718-2511
Search for more papers by this authorMatthieu Faure
Institut de Chimie, Université de Neuchâtel Case postale 2, 2007 Neuchâtel, Switzerland, Fax: (+41) 32-718-2511
Search for more papers by this authorThomas R. Ward Prof. Dr.
Institut de Chimie, Université de Neuchâtel Case postale 2, 2007 Neuchâtel, Switzerland, Fax: (+41) 32-718-2511
Search for more papers by this authorThis work was supported by the Fonds National Suisse de la Recherche Scientifique. A genereous loan of ruthenium chloride by the Johnson Matthey Technology Centre is gratefully acknowledged.
Graphical Abstract
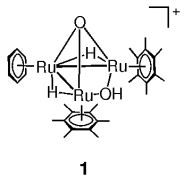
At the interface of homogeneous, heterogeneous, and enzymatic catalysis is the catalytic hydrogenation of benzene to give cyclohexane by the triruthenium cluster 1. Experimental evidence and molecular modeling studies strongly support a catalytic mechanism in which the aromatic substrate is hydrogenated in the hydrophobic pocket spanned by the three η6-bound arene ligands without being coordinated to a Ru center.
References
- 1
- 1a
S. Bhaduri, D. Mukesh, Homogeneous Catalysis, Wiley-Interscience, New York, 2000;
10.1002/0471220388 Google Scholar
- 1b Applied Homogeneous Catalysis with Organometallic Compounds ( ), VCH, Weinheim, 1996 ;
- 1c G. W. Parshall, S. D. Ittel, Homogeneous Catalysis, Wiley-Interscience, New York, 1992.
- 2 B. R. James, Adv. Organomet. Chem. 1979, 17, 319.
- 3 D. Forster, Adv. Organomet. Chem. 1979, 17, 255.
- 4 P. M. Maitlis, A. Haynes, G. J. Sunley, M. J. Howard, J. Chem. Soc. Dalton Trans. 1996, 2187
- 5 W. A. Herrmann, B. Cornils, Angew. Chem. 1997, 109, 1074; Angew. Chem. Int. Ed. Engl. 1997, 36, 1048.
- 6
- 6a
R. Noyori, T. Ojkuma, Angew. Chem. 2001, 113, 41;
10.1002/1521-3757(20010105)113:1<40::AID-ANGE40>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2001, 40, 40;
- 6b M. Yamakawa, H. Ito, R. Noyori, J. Am. Chem. Soc. 2000, 122, 1466;
- 6c D. A. Alonso, P. Brandt, S. J. M. Nordin, Ph. G. Andersen, J. Am. Chem. Soc. 1999, 121, 9580;
- 6d K. Abdur-Rashid, A. J. Lough, R. H. Morris, Organometallics 2000, 19, 2655;
- 6e
D. G. I. Petra, J. N. H. Reek, J.-W. Handgraaf, E. J. Meijer, P. Dierkes, P. C. J. Kamer, J. Brussee, H. E. Schoemaker, P. W. N. M. van Leeuwen, Chem. Eur. J. 2000, 6, 2818.
10.1002/1521-3765(20000804)6:15<2818::AID-CHEM2818>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 7
K. U. Wendt, G. E. Schultz, E. J. Corey, D. R. Liu, Angew. Chem. 2000, 112, 2930;
10.1002/1521-3757(20000818)112:16<2930::AID-ANGE2930>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2812.10.1002/1521-3773(20000818)39:16<2812::AID-ANIE2812>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 8 M. Faure, M. Jahncke, A. Neels, H. Stœckli-Evans, G. Süss-Fink, Polyhedron 1999, 18, 2679.
- 9 M. Faure, Tesuro Vallina, H. Stœckli-Evans, G. Süss-Fink, J. Organomet. Chem. 2001, 621, 103.
- 10 M. A. Bennett, T.-N. Huang, T. W. Turney, J. Chem. Soc. Chem. Commun. 1979, 312.
- 11 M. J. Russel, C. White, P. M. Maitlis, J. Chem. Soc. Chem. Commun. 1977, 427.
- 12
- 12a E. L. Muetterties, F. J. Hirsekorn, J. Am. Chem. Soc. 1974, 96, 4063;
- 12b F. J. Hirsekorn, M. C. Rakowski, E. L. Muetterties, J. Am. Chem. Soc. 1975, 97, 237;
- 12c L. S. Stuhl, M. Rakowski Dubois, F. J. Hirsekorn, J. R. Bleeke, A. E. Stevens, M. L. Muetterties, J. Am. Chem. Soc. 1978, 100, 2405.
- 13 A. F. Borowski, S. Sabo-Etienne, B. Chaudret, J. Mol. Catal. A, in press.
- 14
- 14a I. P. Rothwell, Chem. Commun. 1997, 1331;
- 14b V. M. Visciglio, J. R. Clark, M. T. Nguyen, D. R. Mulford, P. E. Fanwick, I. P. Rothwell, J. Am. Chem. Soc. 1997, 119, 3490.
- 15 L. Plasseraud, G. Süss-Fink, J. Organomet. Chem. 1997, 539, 163.
- 16 Garcia Fidalgo, L. Plasseraud, G. Süss-Fink, J. Mol. Catal. A 1998, 132, 5.
- 17 P. J. Dyson, D. J. Ellis, D. G. Parker, T. Welton, J. Chem. Soc. Chem. Commun. 1999, 25.
- 18 D. Durand, G. Hillion, C. Lassau, L. Sajus, US Patent 4 271 323 1981.
- 19 H. Brunner in Applied Homogeneous Catalysis with Organometallic Compounds ( ), VCH, Weinheim, 1996, p. 216.
- 20 M. P. Gomez-Sal, B. F. G. Johnson, J. Lewis, P. R. Raithby, A. H. Wright, J. Chem. Soc. Chem. Commun. 1985, 1682.
- 21 CSD refcodes FEBLIH, FEBNOP, GEHZIC, HIPKUM, HIPLAT, KIRWEL, PIDVAZ, REKPOM, REKPUS, REPYEQ, REPYIU.
- 22 The extensible systematic force field (ESFF) code was used to optimize the gas-phase geometry of the host–guest complexes [C6D6⊂1]+ and [C6D6⊂2]+. Keeping the cluster geometry frozen, the benzene guest was gradually allowed to approach the open face of the cluster. The total energy was minimized by varying the orientation of the benzene with respect to the {Ru3-face}. See: S. Barlow, A. L. Rohl, S. Shi, C. M. Freeman, D. O'Hare, J. Am. Chem. Soc. 1996, 118, 7578.
- 23 K. S. Weddle, J. D. Aiken III, R. G. Finke, J. Am. Chem. Soc. 1998, 120, 5653.
- 24a J. Schulz, A. Roucoux, H. Patin, Chem. Commun. 1999, 535;
- 24b
J. Schulz, A. Roucoux, H. Patin, Chem. Eur. J. 2000, 6, 618.
10.1002/(SICI)1521-3765(20000218)6:4<618::AID-CHEM618>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 25 P. Foley, R. Di Cosimo, G. M. Whitesides, J. Am. Chem. Soc. 1980, 102, 6713.
- 26 R. M. Laine, J. Mol. Catal. 1982, 14, 137.